MolecuLight i:X & DX may help at every stage of wound care

Assessment
Visualize fluorescent bacteria and measure wound surface area in real-time to understand the status of the wound more fully.1,3
Cleaning
Allows clinicians to focus cleaning in areas where fluorescent bacteria are located and optimize wound bed preparation.10
Debridement
Guides more efficient and targeted debridement.2,6,7
Sampling
Guides where to sample; 54% more accurate swabbing compared to the Levine technique.3
Documentation
Provides objective visual documentation of the presence of fluorescent bacteria and the surface area of the wound.2,9
Treatment Selection
Comparing fluorescent bacteria and wound surface area at each visit may provide real-time objective feedback on impact of treatment plan.1,2,4
Antibiotic Stewardship
Supports more responsible antibiotic decision making and selection.6,8,9
Patient Engagement
Patients easily see and understand why a clinician is taking certain action to clean, debride, and treat a wound in a specific way.4,5
Watch MolecuLight i:X in action
The MolecuLight i:X Wound Imaging Device allows clinicians to quickly, safely, and easily visualize bacteria2 and measure wounds7 at the point of care, so they have maximum insights for accurate treatment selection and accelerated healing.
New peer-reviewed publications featuring MolecuLight appeared in:
Real-Time Detection of Asymptomatic Bioburden in Wounds
Use of the MolecuLight i:X to image for bacterial fluorescence, in conjunction with clinical signs & symptoms, adds an additional bacterial-specific piece of information that can be captured and considered in real-time.
See Full Case Study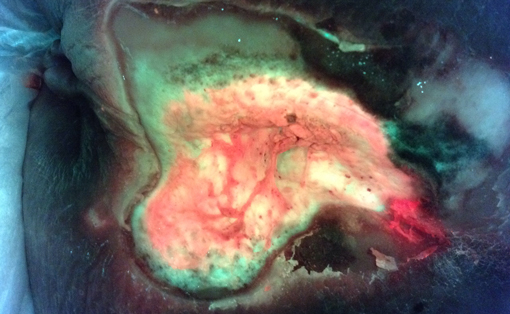
 i:X
i:X
 DX
DX



























