Bacterial colonization in chronic wounds can significantly delay or entirely prevent wound healing.1 Therefore, detection of significant bacterial burden in a wound typically mandates a change in treatment plan towards one which targets bioburden. Real-time detection of bioburden currently relies on subjective, qualitative visual assessment of clinical signs and symptoms (e.g. pain, exudate, crusting, swelling, erythema, foul odour, friable granulation tissue, and heat). Yet high levels of bacteria often occur in the absence of signs and symptoms, even some cases of wound infection.1-3 Without real-time confirmation of the presence and spatial distribution of bacterial burden within and around a wound, clinicians are at risk of making inappropriate and ineffective treatment decisions.

Clinician's Testimonial
"The MolecuLight i:X gives clinicians the ability to visualize and therefore target treatment towards clinically undetectable critical colonization. This user friendly point-of-care device is an exciting addition to our wound care protocol."
David Russell, MD, Leeds General Infirmary, Leeds, UK
Clinician Profile
David Russell, MD is a consultant vascular surgeon at Leeds Teaching Hospitals NHS Trust, located in Leeds, West Yorkshire, UK.
Clinical Synopsis
Patient Condition: 60 year old patient with diabetic foot ulcer (> 5 months) on left plantar heel. Patient exhibited no clinical signs or symptoms of infection, received regular debridement, and offloaded the wound via a total contact cast, yet the wound was deteriorating rapidly.
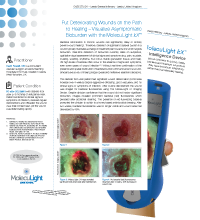
This diabetic foot ulcer patient had significant wound deterioration (3-fold area increase over 4 weeks) despite aggressive offloading, good vascularity, and no clinical signs or symptoms of infection. After routine debridement the wound was imaged for bacterial fluorescence using the MolecuLight i:X Imaging Device. Despite clinician confidence that the wound did not harbor significant bioburden, images revealed prominent bacterial (red) fluorescence which persisted after additional cleaning. The presence of red-fluorescing bacteria prompted the clinician to switch to a honey-based antimicrobial dressing.
 Diabetic foot ulcer with significant deterioration (3x area increase over 4 weeks) |
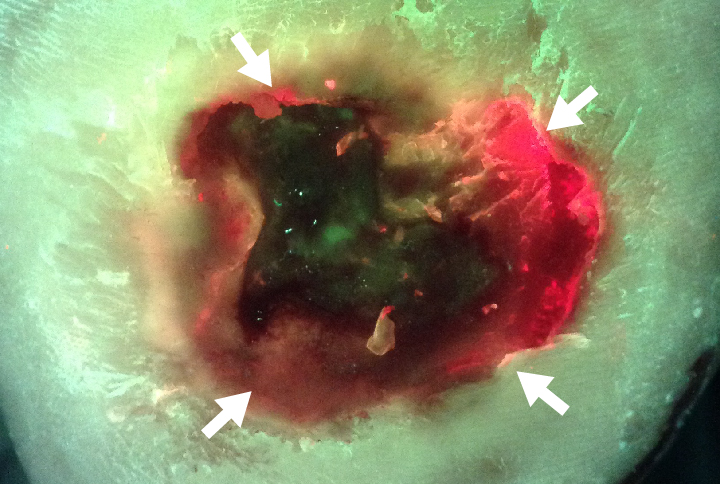 FL-image revealed significant bioburden (red fluorescence) after debridement |
|
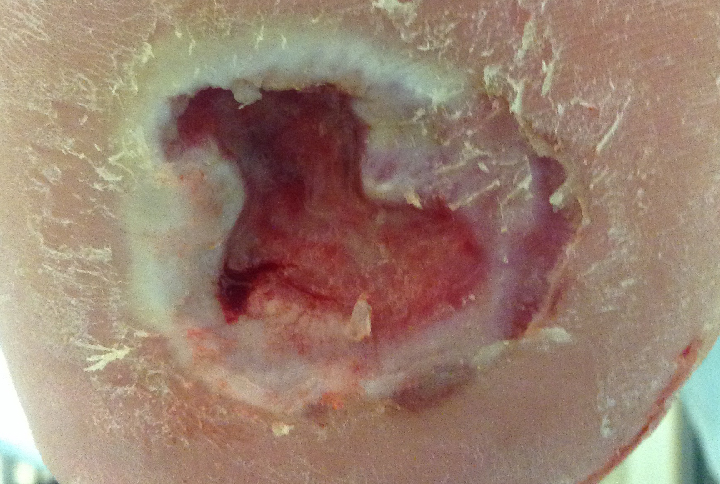
However, after two weeks on this optimized treatment plan, this previously deteriorating wound was on the path to healing. The wound area of this diabetic foot ulcer had decreased by 45% and bacterial fluorescence was no longer visible (Figure 4).
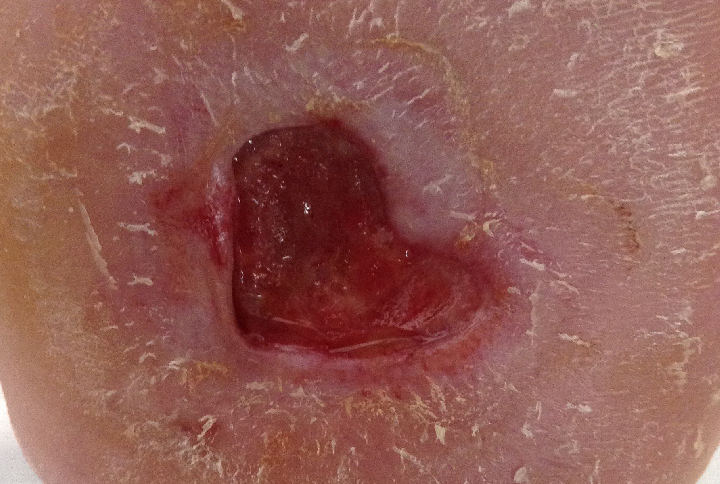 After 2 weeks, wound area had decreased by 45% |
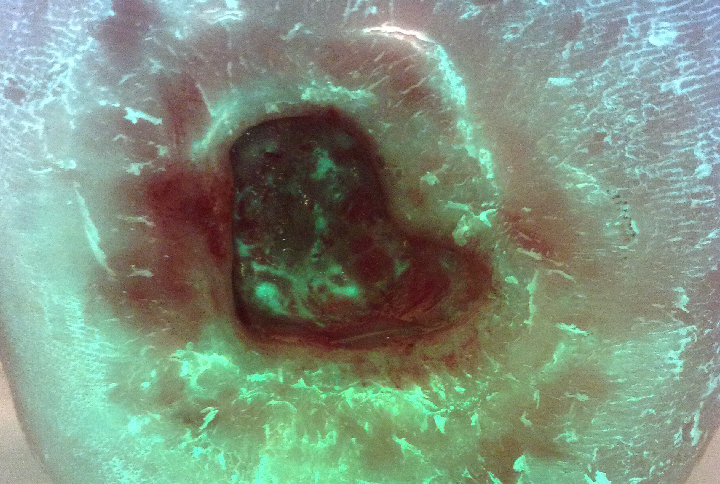 After 2 weeks, no red fluorescing bacteria observed |
|
At a Glance
| Wound etiology & location | Diabetic foot ulcer (5 months), left plantar heel |
| Patient demographics | Male, 60 years old |
| Patient-related challenges | Deteriorating wound (area increased 3-fold over 4 weeks) despite aggressive offloading and good vascularity |
| Patient’s general care paradigm |
Total contact cast Regular debridement |
| Clinician stated utility of the MolecuLight i:X |
Detection of asymptomatic bioburden Guided antimicrobial dressing selection |