Results from 211 Facilities Show MolecuLight Imaging is a Valuable Tool in Improving Bacterial Infection Management
TORONTO, ON – (May 23, 2023) MolecuLight Inc., the leader in point-of-care fluorescence imaging that locates and detects elevated, pathogenic bacterial loads in and around wounds, announced the publication of their latest publication, “Lights, fluorescence, action—Influencing wound treatment plans including debridement of bacteria and biofilms ”1 in the International Wound Journal. This represents the largest wound imaging study of real-world evidence. It describes single-timepoint treatment decisions made on 1,000 chronic wounds at 211 wound-care facilities across 36 US states.
Management of wound bacterial loads, including biofilm, is paramount to the prevention of infection and its severe and costly complications. During a patient exam, clinicians rely on various tools to guide their treatment decisions; however, some of these tools can be subjective. To address this issue, fluorescence imaging with MolecuLight provides a valuable, objective tool for wound assessment at the bedside, filling a significant gap in current clinical practice.
In the study, clinicians initially evaluated the wounds based on clinical signs and symptoms and documented a treatment path. The wounds were then imaged using MolecuLight i:X® or DX™ devices to identify the presence and location of harmful bacterial loads. The study revealed that fluorescence signals indicating elevated bacterial loads were observed in 70.8% of the wounds, whereas only 29.6% showed clinical signs and symptoms of infection. As a result, a significant percentage of wounds with harmful bacterial levels were asymptomatic and lacked clinical signs and symptoms that could have been missed by clinicians. Following fluorescence imaging with MolecuLight, clinicians used objective information on the wound’s bacterial status to adjust the initially outlined treatment plan(s) in over 50% of the wounds (528/1000). Some of the treatment changes included:
- More extensive debridement (18.7%),
- More extensive hygiene (17.2%),
- Fluorescence-targeted debridement (17.2%),
- New topical therapies (10.1%),
- Fluorescence-guided sampling for microbiological analysis (6.2%), and
- Changes in dressing selection (3.2%).
These findings align with previous clinical trial results using MolecuLight and illustrate that point-of-care imaging information improves bacterial infection treatment planning and management. The study highlights the value of MolecuLight devices as objective diagnostic tools for identifying pathogenic bacteria and informing clinical decision-making in wound care.
“Most chronic wounds with high bacterial loads fail to mount symptoms of infection, making detection and treatment very difficult for the clinician”, says Dr. Robert Skerker, a co-author and Medical Director, Wound Healing Center at Morristown Medical Center in Morristown, NJ. “As the study shows, the MolecuLight device now gives wound care clinicians an objective bedside diagnostic tool that provides instant information on the presence of troublesome bacteria to immediately inform our clinical decision-making. MolecuLight has become an invaluable tool in our clinic and we continue to use the imaging results to support decisions that are helping us improve patient outcomes”.
Dr. Raymond Abdo, a co-author and podiatrist at St. Louis Foot & Ankle in St. Louis, MO, explains that using the MolecuLight device to image wounds is highly beneficial for customized treatment plans. “It reveals detrimental bacterial loads that would otherwise be invisible. This is information we need for optimizing treatment plans for a wound, which it frequently does. Additionally, the device’s advanced digital wound measurement feature enables accurate and consistent documentation of patients’ wound measurements and healing progress, alerting us to any healing delays. This seamlessly integrates into the workflow, without hindering my clinic’s busy patient workload”.
In addition to these benefits, a recent publication “Skin Pigmentation Impacts the Clinical Diagnosis of Wound Infection: Imaging of Bacterial Burden to Overcome Diagnostic Limitations”2 showed that imaging using MolecuLight also has great clinical potential to serve as a more objective and equitable indicator of wound bacteria across all skin tones.
The MolecuLight i:X and DX are the only imaging devices for the real-time detection of elevated bacterial burden in wounds that are FDA cleared and CE and Health Canada approved. With clinical evidence including over 80 peer-reviewed publications involving 2,600 patients, they are used by leading wound care facilities globally.
References
1Jacobs, A. et al, Lights, fluorescence, action—Influencing wound treatment plans including debridement of bacteria and biofilms, Int Wound J. 2023;1–10.
2Johnson, J. et al. Skin Pigmentation Impacts the Clinical Diagnosis of Wound Infection: Imaging of Bacterial Burden to Overcome Diagnostic Limitations. J. Racial and Ethnic Health Disparities (2023).
About MolecuLight Inc.
MolecuLight Inc., is a privately-owned medical imaging company that has developed and is commercializing its proprietary fluorescent imaging platform technology in multiple clinical markets. MolecuLight’s commercial devices, which include the MolecuLight i:X® and DX™ fluorescence imaging systems and their accessories, are point-of-care handheld imaging devices for the real-time detection and localization of bacterial load in wounds and digital wound measurement. MolecuLight procedures performed in the United States benefit from an available reimbursement pathway which includes two CPT® codes for physician work to perform “fluorescence imaging for bacterial presence, location, and load” and facility payment for Hospital Outpatient Department (HOPD) and Ambulatory Surgical Center (ASC) settings through an Ambulatory Payment Classification (APC) assignment. The company is also commercializing its unique fluorescence imaging platform technology for other global markets with relevant unmet needs in food safety, consumer cosmetics and other key industrial markets.
For more information, contact:
Rob Sandler
Chief Marketing Officer
MolecuLight Inc.
T. +1.647.362.4684
rsandler@moleculight.com
www.moleculight.com
Images:
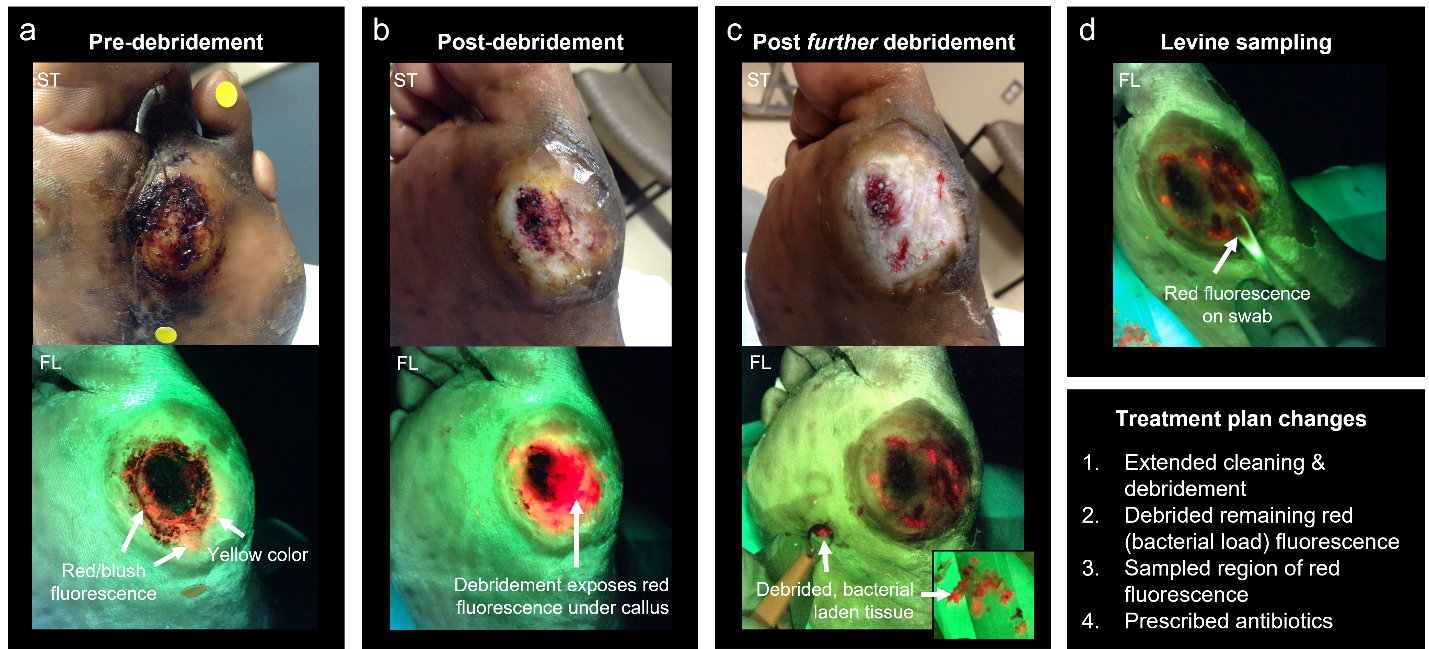
Caption: Fluorescence-informed treatment plan changes using MolecuLight imaging for a stalled diabetic foot ulcer (DFU) exhibiting delayed healing but no signs or symptoms of infection. Standard (ST) and fluorescence (FL) images (MolecuLight) were captured (a) after gentle cleansing to remove blood/ surface debris, (b) following curettage debridement to address the callus, (c) following further curettage debridement to address the bright red fluorescence revealed upon initial debridement, and (D) during Levine swab sampling of an area of remaining red fluorescence for microbiological analysis. Red fluorescence signal indicates the presence of bacteria at elevated loads >104 CFU/g.
Attached and download at: https://moleculight.box.com/s/933ixn6iufbgzg8g5750brvl4v1eauvn










