The new era of point-of-care testing
The March 2016 Issue of the Medical Post Magazine brings an article on ‘The new era of point-of-care testing. The latest devices are very different from those of the past. The big question is: will physicians use them?’ by Tristan Bronca.
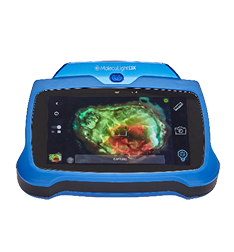
“… Recently, Health Canada approved a device called MolecuLight i:X, whose design was based on an unexpected observation made in 2007: bacteria glow. Dr. Ralph DaCosta (PhD), a scientist with the Princess Margaret Cancer Centre and the University Health Network’s Techna Institute in Toronto, was able to develop a sort of camera/flashlight out of technology no more complicated than that in a smartphone.
It could detect “clinically significant” amounts of bacteria in wounds by calibrating out normal bacteria found on the skin. Without spraying any contrast agents or even touching the wound, infections lit up.
The standard of care in wound care is you smell it, you look at it, you assess whether there’s pus or blood or other signs and symptoms,” explained Dr. DaCosta. Clinicians use highly subjective methods to determine whether the wound is infected.
It was an area that he thought was screaming for new methods and tools. Not only did the new device offer an objective measurement, it also affected patient outcomes. One study published in PLOS One (March 2015) found that over a six-month trial, diabetic foot ulcers closed three times faster with the use of the light versus standard care. In a case study published in the International Wound Journal (April 2015), the device was used to detect a subsurface infection in an asymptomatic patient who was about to be discharged from hospital.
Earlier this year, Dr. DaCosta launched a company, MolecuLight Inc., to market fluorescence guided wound imaging technologies, and currently about 20 practitioners in Ontario are using the first-generation device…”