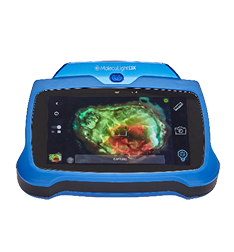
MolecuLight i:X wound care device instantly visualizes potentially harmful bacterial loads
TORONTO – September 16, 2016 – MolecuLight Inc. has received CE Mark approval authorizing commercial sales in the European Union of its MolecuLight i:X Imaging Device, that is revolutionizing wound care.
The MolecuLight i:X will be making its European debut at the World Union of Healing Societies (WUWHS) World Congress, September 25-29 in Florence, Italy. The company will introduce the product to wound care clinics and hospitals across Europe, demonstrating how, with the MolecuLight i:X incorporated into their clinical assessment of wound care patients, health care professionals will have access to real-time visualization of potentially harmful bacteria immediately at the bedside.
The MolecuLight i:X uses the principle of fluorescence to capture and document either still images or videos of wounds as well as their surrounding areas where potentially harmful bacteria may be present, without the need of contrast agents.
“This CE Mark approval is a major milestone for both our company and wound care as a whole,” says Craig Kennedy, MolecuLight’s CEO. “With our device already approved for use in Canada and now available in the EU, we believe that in time the MolecuLight i:X will become the stethoscope of wound care worldwide. We are eager to begin our commercialization efforts in Europe starting with the WUWHS Congress in Florence later this month.”
Dr. Ralph S. DaCosta, Founder, Chief Scientific Officer and Board Director adds: “By helping clinicians see potentially harmful bacteria levels in real-time at the bedside, we are advancing wound healing by providing a more personalized and effective approach. Soon, we will be including additional functionality to also easily and quickly measure wound size. All integrated on the same device.”
About MolecuLight Inc.
Founded in 2012, and based in Toronto, Canada, MolecuLight Inc. is a privately-owned medical imaging company delivering fluorescence image-guidance solutions that provide clinicians with new information about wound bacterial burden for better diagnostic and treatment decisions.
MolecuLight strives to become the ubiquitous name in fluorescence-guided wound imaging technologies and to serve the healthcare industry with world-class products and services while delivering value to all customers, employees and shareholders.