Published results provides guidelines to standardize use of fluorescence imaging among wound care providers to enhance the quality of patient care
TORONTO, CANADA – (July 7 2021) MolecuLight Inc., the leader in point-of-care fluorescence imaging for real-time detection of wounds containing elevated bacterial loads, announced the publication of an independent consensus study of 32 leading wound care clinicians in Diagnostics 1. The publication, “Guidelines for point-of-care fluorescence imaging for detection of wound bacterial burden based on Delphi consensus”, presents the results of a Delphi consensus used to establish agreement on guidelines describing the use of fluorescence imaging for detection of elevated bacterial burden in wounds. In the study, a two-round Delphi process was employed amongst a multidisciplinary panel of 32 wound experts across multiple sites of service including hospital outpatient, inpatient, private office and long-term care residences. The result was that 96% of clinicians reported that treatment plans informed by the MolecuLight procedure led to improved wound healing. The agreed-upon guidelines will help to standardize the use of fluorescence imaging using the MolecuLight i:X among wound care providers and enhance the quality of patient care.
In addition to guidelines on when and how frequently the MolecuLight imaging procedure should be performed, notable findings describing the impact of the MolecuLight i:X on clinical experiences include:
- >80% of clinicians reported changes in treatment plans,
- 96% reported that imaging-informed treatment plans led to improved wound healing,
- 78% reported reduced rates of amputations, and
- 83% reported reduced rates of microbiological sampling.
“The results of the Delphi survey show an incredibly high level of agreement (>85%) across a wide variety of leading wound care professionals”, says Dr. Thomas Serena, the publication’s senior author and Founder and Medical Director of The SerenaGroup®. “This indicates strong support for the adoption of these clinical indications around the MolecuLight i:X point-of-care fluorescence imaging system”.
“The high level of agreement amongst so many experts demonstrates the utility and value of this point-of-care imaging technology in the management of chronic wounds”, say Martha R. Kelso RN, LNC, HBOT, contributing author of the publication, and Founder and Chief Executive Officer of Wound Care Plus, LLC. “The use of this imaging procedure enables healthcare providers to rapidly detect elevated levels of bacteria and to develop an effective treatment plan while at the same time avoiding the use of advanced therapies when they are contradicted. In addition to improving patient outcomes, this technology has high potential to reduce the overuse of antimicrobials and reduce healthcare costs for both patients and health systems”.
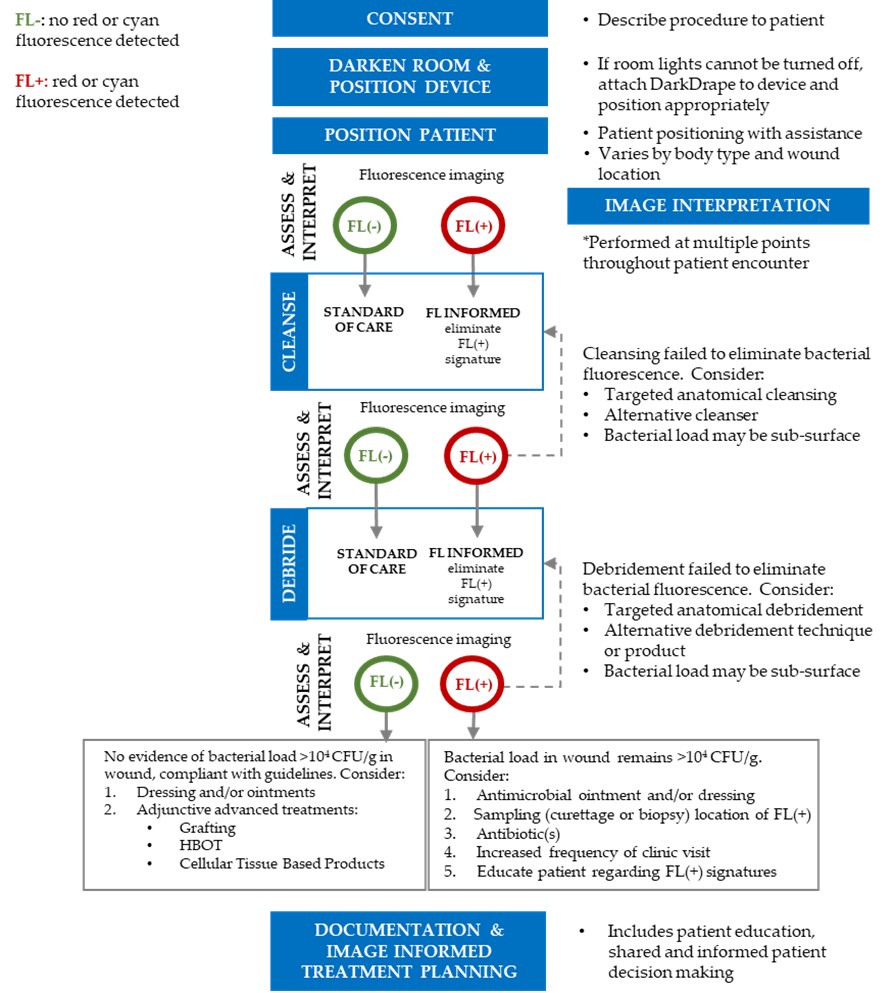
A key outcome of the Delphi consensus was the development of a clinical workflow for fluorescence imaging of elevated bacterial loads. This workflow describes how to most effectively leverage imaging of bacterial burden to enhance wound hygiene and better inform treatment planning based on objective point-of-care information on wound bioburden. It also serves to improve the consistency of care across the wound care continuum.
Workflow describing how to use the MolecuLight procedure
during a patient visit to inform wound treatment planning
Authors of the publication will participate in a panel discussion webinar entitled “When should I perform point-of-care fluorescence imaging of wound bioburden? Guidelines based on Delphi consensus”. In this session, authors will review the study findings and discuss why they believe fluorescence imaging of elevated bacterial burden is an essential procedure in their wound care. The webinar, hosted by WoundSource, will be held on July 27, 2021 at 1:00 pm EST. To register, visit https://tinyurl.com/58kk2xyr.
References
1 Oropallo, A.R et al, Guidelines for Point-of-Care Fluorescence Imaging for Detection of Wound Bacterial Burden Based on Delphi Consensus, Diagnostics 2021, 11, 1219
About MolecuLight Corp.
MolecuLight Inc., a privately-owned medical imaging company that has developed and is commercializing its proprietary fluorescent imaging platform technology in multiple clinical markets. MolecuLight’s first commercially released device, the MolecuLight i:X fluorescence imaging system and its accessories provide a point-of-care handheld imaging device for the global wound care market for the detection of wounds containing elevated bacterial burden (when used with clinical signs and symptoms) and for digital wound measurement. The company is also commercializing its unique fluorescence imaging platform technology for other markets with globally relevant, unmet needs including food safety, consumer cosmetics and other key industrial markets.
Rob Sandler
Chief Marketing Officer
MolecuLight Inc.
M. +1.416.274.8166
rsandler@moleculight.com










