ABSTRACT
Introduction:
Amputations amount to $8.3 billion in hospital costs annually in the United States.1 In the general population of England more than 2700 lower limb amputations occur annually, with a mortality rate of 17%.2 Infection is a major potential complication in all wounds, however the highest rate of surgical site infection is associated with lower limb amputations,3 adding to the cost and patient quality of life degradation in this population. Advanced therapies such as skin grafting are often used to close these wounds. Deciding when a wound is ready for grafting presents a clinical challenge, as grafts are contraindicated when bacteria is present, costly to the healthcare institution, and very likely to fail in the event that significant bacterial burden was present in the wound.4,5 Diagnosing high levels of bacteria or infection based on traditional clinical signs and symptoms is difficult as bacteria are invisible to the unaided eye. To address this problem, bacterial fluorescence imaging can be used to visualize concerning levels of bacteria in real-time at the bedside using a non-contact hand held device.6,7 Herein, we report how detection of asymptomatic bacterial presence in an amputated stump with this device helped prevent an unnecessary skin graft surgery, resulting in significant cost savings and improved patient outcome.
Methods:
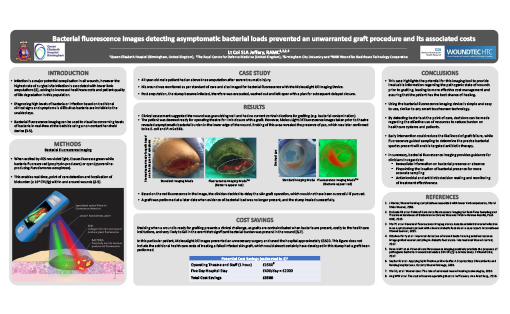
47-year-old male patient had an above knee amputation after severe traumatic injury. His wound was monitored as per standard of care and also imaged for bacterial fluorescence with the MolecuLight i:X Imaging Device, which enables real-time visualization and localization of bacteria (loads ≥ 104 CFU/g)7 within and around wounds. The presence of bacterial fluorescence led to adjustments in treatment plan. Fluorescence images were used to guide wound sampling to verify presence of pathogens.
Results:
Post amputation the stump became infected, therefore was evacuated, washed out and left open with a plan for subsequent delayed closure. One week later, clinical assessment suggested the wound was granulating well and had no current contraindications for grafting (e.g. bacterial contamination). The patient was deemed ready for operating theatre for limb closure with a graft. However, MolecuLight i:X fluorescence images taken prior to theatre revealed bacterial burden in the lower edge of the wound. Fluorescence-guided probing of this area revealed the presence of pus, which was later confirmed to be E. coli and P. mirabilis. Based on this information, the clinician decided to delay the skin graft operation, resulting in approximately £3500 in direct cost savings. This figure does not include the additional health care costs of treating a failed infected skin graft, which would almost certainly have developed in this stump had a graft been performed. A graft was performed at a later date when evidence of bacterial load was no longer present, and the stump healed successfully.
Conclusion:
The use of the MolecuLight i:X Imaging Device guided appropriate intervention for a patient that would otherwise have been closed with a clinically significant pathogenic load, resulting in significant cost savings and improved patient outcome. This case highlights the potential for this imaging tool to provide invaluable information regarding the pathogenic state of wounds prior to grafting, leading to more effective cost management and assuring that the patient has the best chance of healing.