Abstract:
Introduction: Wound microflora in hard-to-heal wounds is invariably complex and diverse. Determining the interfering organisms(s) is therefore challenging. Tissue sampling, particularly in large wounds, is subjective and, when performed, might involve swabbing or biopsy of several locations. Fluorescence (FL) imaging (MolecuLight) of bacterial loads is a rapid, non-invasive method to objectively locate microbial hotspots (loads >104 CFU/gr). When sampling is deemed clinically necessary, imaging may indicate an optimal site for tissue biopsy. This study aimed to investigate the microbiology of wound tissue incisional biopsies taken from sites identified by FL imaging compared with sites selected by clinical judgment.
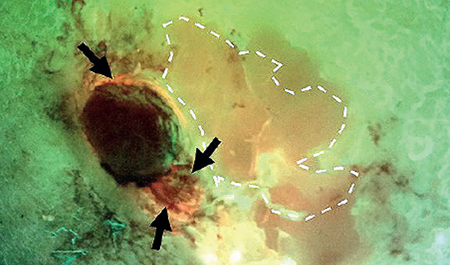
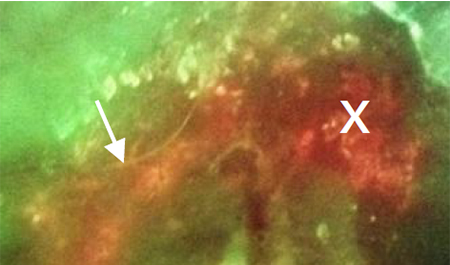
Methods: A post hoc analysis of the 350-patient FLAAG wound trial was conducted; 78 wounds were included in the present study. All 78 wounds were biopsied at two sites: one at the center of the wound per standard of care (SoC) and one site guided by FL-imaging findings, allowing for comparison of total bacterial load (TBL) and species present.
Results: The comparison between the two biopsy sites revealed that clinical uncertainty was higher as wound surface area increased. The sensitivity of a FL-informed biopsy was 98.7% for accurately finding any bacterial loads >104 CFU/g, compared to 87.2% for SoC (p=0.0059; McNemar test). Regarding species detected, FL-informed biopsies detected an average of 3 bacterial species per biopsy versus 2.2 species with SoC (p < 0.001; t-test). Microbial hotspots with a higher number of pathogens also included the CDC’s pathogens of interest.
Conclusions & perspective: FL imaging (MolecuLight) provides a more accurate and relevant microbiological profile that guides optimal wound sampling compared to clinical judgment. This is particularly interesting in large, complex wounds, as evidenced in the wounds studied in this post hoc analysis. In addition, fluorescence imaging enables earlier bacterial detection and intervention, guiding early and appropriate wound hygiene and potentially reducing the need for antibiotic use. When indicated, this diagnostic partnership with antibiotic stewardship initiatives is key to ameliorating the continuing threat of antibiotic resistance.